One of the most dangerous infectious diseases in cats is borreliosis, the consequences of which can be the most unfavorable for both animals and people.
The disease is seasonal and widespread throughout the Russian Federation, especially in wooded and wooded areas where natural carriers live - wild animals and ticks. Both domestic animals and even people participate in the biological chain of its spread.
However, there is still a very vague understanding of the causative agents of the disease, symptoms, prevention and treatment of borreliosis, and awareness, in this case, is of great importance.
ETIOLOGY OF BORRELIOSIS IN DOGS
Lyme borreliosis is a current zooanthroponosis.
Tick-borne spirochetoses are a group of diseases that affect humans and animals throughout the world.
They can be divided into the Lyme disease group (Lyme borreliosis)
, which is transmitted by ixodid ticks, and the relapsing fever group, which is transmitted by soft ticks (argasids). The Lyme disease group has only recently been recognized and is now the most commonly diagnosed vector-borne disease in humans. Borryliosis has been known in Europe since the 1900s; Alan Steere of Yale University first described the disease and its association with ticks after an outbreak in 1975 in Lyme, Connecticut (after which Lyme disease was named). However, the clear suggestion is that it is a native wildlife infection and its tick vectors are much older. Research showing that ear skin samples from a white-footed hamster species museum collected near Denis, Massachusetts, in 1894 contained Borrelia DNA. Borrelia microorganisms were identified in British ticks in 1897. Lyme disease is now observed in the North. America, Europe and Asia, unconfirmed cases in Australia, South America and Africa. Experimentally induced and naturally occurring Lyme borreliosis has been described in dogs, cats, and other domestic animals. The amount of antibodies in dogs in endemic areas correlates with time spent in the forest and in the city and time outside. In some endemic areas (eg southern Germany), about 30% of ticks are infected and approximately 95% of dogs have antibodies indicating infection.
Lyme disease is caused by Borrelia burgdorferi
and includes several species that affect humans and animals throughout the world. Most similar to a spirochete, Borrelia are small, corkscrew-shaped, motile, microaerophilic bacteria of the order Spirochaetales that can move through connective tissue using flagella. At least 6 species share common properties between dogs and humans. B. b. sensu stricto is dominant in humans and dogs in the United States. It is also found in Europe, but in only 10% of isolates. Borrelia gorinii and Borrelia afzelii are the most common species in Europe. B. japonica is isolated from ticks collected from humans and dogs in Japan. Recently, 2 new species B.lusitaniae and B.valaisiana were discovered. In cats, the natural disease is poorly documented, although cats can be experimentally infected. Cats appear to show less development of clinical signs than dogs, and dogs are less sensitive than humans.
Presence of disease caused by Borrelia
, and its clinical signs depend on the isolate in a given geographic region. Genetic variability among isolates also occurs within each continent. In humans, different species are clearly associated with different tissue tropisms and clinical problems; probably also in dogs. Borrelia burgdorferi sensu stricto infection is associated with round skin lesions, polyarthritis and meningitis. Meningopolyneuritis (Bannwart syndrome) is the primary clinical sign of Borrelia gorinii infection in humans in Europe, while Borrelia afzelii infection is associated with chronic arthritis and dermatitis (erythema chronicum migrans).
Stages of the disease
The development of borreliosis includes three successive stages, preceded by an asymptomatic incubation period. The division into stages is arbitrary, as it describes the clinical picture of the disease, which may differ in different patients, and sometimes be absent. In some patients, the disease manifests itself only in the first stage, in others - in the second and third.
We recommend reading: Pigeon bumps on the head
Incubation period
Like most infections, borreliosis does not manifest itself immediately. Its incubation period usually ranges from 3 to 30 days. The first symptoms most often appear when the wound from a tick bite has long healed.
1st stage
The early localized stage of the disease is the period of time when bacteria have not yet spread throughout the body. The stage is characterized by the presence of primary effects on the skin (rash - erythema migrans), which disappear after a few days or weeks, sometimes months. Antibodies to Borrelia may not yet be detected in the patient’s blood during this period.
2nd stage
After the first stage, which is accompanied by a recognizable rash, the disease seems to go away, all symptoms disappear, sometimes for several weeks. However, if a person remains untreated at this time, the infection will reappear and an early disseminated stage will occur. This means that bacteria have already begun to spread through the blood and lymph to the organs. At the second stage, usually 4-5 weeks (sometimes later) after infection, the first signs of infection affecting the nervous and cardiovascular systems begin to appear.
3rd stage
The late disseminated stage corresponds to the period when bacteria have already spread throughout all systems and organs.
The third stage develops several months or years after infection and cannot appear earlier than the previous two stages. At the same time, its symptoms may be the first if the disease did not manifest itself before. The main symptom of the third stage of borreliosis is arthritis - joint damage.
Chronic ixodic tick-borne borreliosis
Chronic Lyme disease is referred to by some practitioners as post-Lyme disease syndrome. Although most patients with borreliosis can be cured with a 2-4 week course of antibiotics, some patients may continue to have symptoms (joint pain, fatigue, confusion) many years after the illness. This development of infection is usually associated with an autoimmune reaction of the body.
The term chronic borreliosis is also used to describe symptoms in people without clinical or diagnostic evidence of current or past infection with Lyme disease. Due to the lack of a clearly defined clinical definition of "chronic borreliosis", many experts do not support its use.
PATHOGENESIS OF BORRELIOSIS IN ANIMALS AND HUMANS
Unlike Leptospira, Borrelia do not survive in a free environment. They are host-associated and transferred between vertebrate reservoir hosts and blood-sucking arthropods. The main vectors of Borrelia burgdorferi sensu lato are various species of ixodid ticks. Y. ricinus and Y. persulcatus are the main vectors in Europe and Asia and China, respectively. In the United States, black-legged ticks Y. scapularis (Northeast, Midwest, Southeast), Y. pacificus (West) are the main vectors. These small ixodid ticks (less than 3 mm) most often feed on more than one host during their life cycle.
Y. scapularis is a three-host tick with a two-year life cycle. The infected nymph overwinters and in the spring transmits the infection to the reservoir host, which returns the infection by feeding the larvae. Larvae and nymphs feed initially on rodents and small mammals (northern Y. scapularis) and reptiles (southern Y. scapularis), while adult ticks feed on deer and other large mammals. People and pets are usually infected by nymphs or adult ticks. Because reptiles are poor reserve hosts, infection rates in southern Y. scapularis are much lower than in northern ones. Moreover, since southern ticks do not feed on mammals, the presence of Borrelia infection is relatively lower in southern regions. Other ticks and insect vectors can harbor Borrelia. But they are not the main source of infection and an important vector. Direct transfer of spirochetes is not possible, since it is transovarial transfer in ticks. Dog urine is also not a suitable source of spread. In a natural infection model, control dogs kept with infected dogs for a year did not show an increase in antibodies and microorganisms were not isolated from the urine of infected dogs. These studies also do not document any incidence of intrauterine transmission. However, Borrelia can survive freezing and storage, making artificial insemination a potential source of contamination. Blood transfusion is another potential source of infection.
Natural transmission of spirochetes takes 48 hours from tick attachment, during which the m/o multiply and pass through the intestinal epithelium into the hemolymph, colonize the salivary glands and infect the host with tick saliva. Once in the body, spirochetes usually cause persistent infection. Obvious experimental suggestions exist that m/o Borrelia is a strictly extracellular parasite and, in an unclear way, can escape immune elimination. M/s can reside and germinate for a long period (possibly throughout the life of most animals) in the intercellular spaces, in the skin at the site of a tick bite. Most infected animals never show clinical signs. In dogs that do not show clinical signs, Borrelia probably persists but does not affect the connective tissue and therefore does not cause clinical signs, but may cause persistent antibodies. In very few animals, Borrelia multiply and migrate from the skin of the bite site through the connective tissue, including joints, starting with those closest to the bite site. Clinical manifestations of the disease in these dogs lead to an inflammatory response in the owner to their presence and migration. Despite treatment, spirochetes may persist for several months or years in the skin, connective tissues, joints, and nervous system and can be detected by PCR, or sometimes by blood, CSF, or urine culture.
It is not yet known why a particular animal or person begins to show clinical signs. The pathogenesis depends on the Borrelia species and B. burgdorferi sensu stricto appears to be more pathogenic in dogs than the species found mostly in Europe. It is estimated that 5-10% of dogs with antibodies in endemic areas in the United States show clinical signs of disease within 2 to 5 months after infection, with a much smaller percentage in endemic areas in Northern Europe. Experimental studies have shown that the number of infected ticks that feed is limited. The age and immune status of the animal is also important, and treatment with high doses of corticosteroids during infection increases the likelihood of clinical signs. The development of immune complications, such as arthritis, is probably associated with host immunodeficiency. People with a certain major histocompatibility complex haplotype are prone to more severe manifestations of the disease.
If the cardiovascular system is damaged
When Borrelia attacks the heart muscle, symptoms associated with rapid breathing and heart palpitations may be observed. Since in general the animal body is usually quite resistant to the pathogen, the symptoms of borreliosis in dogs that are classified as cardiac may go unnoticed.
The infection will “lurk” in the body for a long time until it causes a more significant complication - inflammation of the heart muscle. Depending on how severe the pathological process is, you can observe arrhythmia, pulmonary edema, cyanosis of the mucous membranes, as well as other symptoms of heart failure in the animal.
CLINICAL SIGNS OF BORRELIOSIS IN DOGS
In all experimental infections of dogs, the strain Borrelia burgdorferi sensu stricto
Therefore, the clinical condition caused by this microorganism is relatively well described. In experimentally infected dogs, clinical disease begins within 2-5 months. after a tick bite. Clinical signs include fever, lack of appetite, lethargy, lymphadenopathy, and occasional lameness due to polyarthritis. Arthritis begins first in the joint closest to the site of the tick bite. This is observed because pro-inflammatory cytokines are released and interleukin-8 (it-8) plays an important role in the pathogenesis of acute arthritis. Despite the passing (transit) nature of arthritis, pathological changes in the joints progress; Chronic non-erosive arthritis is the primary condition after prolonged infection and this may persist despite antimicrobial therapy.
Clinical signs that develop from natural infections or infections caused by other Borrelia species are less well defined. Glomerulomhritis and protein-losing glomerulopathies progressing to renal impairment have been described in naturally infected dogs. Acute progressive renal failure associated with azotemia, uremia, protenuria, peripheral edema and body cavity effusion was described in 49 dogs with positive endemic Borrelia antibody tests.
Labradors and golden retrievers are most commonly affected. In Europe, glomerulonephritis is often found in Bury Mountain dogs with high titers of antibodies to Borrelia. Borrelia is likely, but not yet proven, to cause rapid progression of glomerular disease. Dermatological lesions due to expanding erythema around the site of the tick bite have not been well documented. Small reddish lesions, not as dramatic as the erythema migrans seen in humans, may be present but disappear within the first week. However, m\o can be released from the surface of the skin for a long period. Neurological manifestations caused by meningitis or encephalitis have not been well documented in other experimental or natural infections of dogs. In addition to arthritic syndromes, cardiac arrhythmias and myocarditis have been noted, similar to reports in humans.
There are no specific hematological or biochemical changes associated with Lyme disease. Unlike leptospirosis, leukocytosis is not observed, possibly due to the fact that Borrelia rarely spread hematogenously, but more often through connective tissue. Dogs with side effects may have proteinuria due to glomerulonephritis, sometimes secondary azotemia, hematuria, pyuria, cylindruria; analysis of synovial fluid shows a typical purulent polyarthritis with a leukocyte level from 2 to 100 thousand per microliter.
If the musculoskeletal system is affected
A common sign of borreliosis in dogs is joint damage. This pathological process occurs in the majority of sick individuals (about 80%). Signs of a pathological condition can be recognized by pain and swelling in the joints (or one of them), alternating lameness on different paws, occurring in periods of three to four days. Sometimes these attacks can be interspersed with “healthy” intervals of several weeks.
The dog loses its appetite, gets tired quickly, does not want to move and is depressed. All this often coincides with the beginning of the next exacerbation of lameness.
These symptoms may occur separately or in combination. They can most often be observed 1-6 months after the tick bite. Diagnosis due to this can sometimes be very difficult.
DIAGNOSIS OF BORRELIOSIS IN DOGS
Lyme borreliosis
, apparently, is overdiagnosed in humans and veterinary medicine as it has become a fashionable disease. Overestimation occurs from antibody cross-reactivity to other infectious agents, from the inaccuracy of antibody tests, and from the dominance of asymptomatic infection. The presence of antibodies to Borrelia means indicators to spirochetes, but it is not certain that the course of the disease is caused by Borrelia. In endemic areas, the majority of animals in the population have antibodies without developing clinical signs.
Various problems have been reported with tests for antibodies to tick-borne borelliosis. First, there is no uniform standardization for antigen preparations, techniques, and interpretations among laboratories. When comparing sera sent to 10 commercial laboratories for antibody testing, only 53% agreement was found. Antibody screening procedures developed for animals in Elisa and ELISA typically use whole cells that contain proteins cross-reactive to other bacteria, especially other spirochetes. In humans, inflammatory conditions such as autoimmune diseases, rheumatoid arthritis, syphilis, periodontal disease and suspected oral spirochetosis show positive results. Leptospirosis - positive canine serum may also have low levels of B. burgdorferi reactivity in Elisa or ELISA. In the UK, dogs with periodontal disease and oral spirochetosis show high levels of antibodies against B. burgdorferi in contrast to healthy dogs in whole cell Elisa or ELISA. Dogs vaccinated against borreliosis show antibodies in whole cell Elisa or ELISA for months to years after vaccination.
Paired titers are not useful in diagnosing Lyme disease because high antibody titers usually persist for a long period. Simultaneous measurement of Yg G and Yg M in one sample could theoretically provide more information about other diseases. However, in naturally infected dogs and people, Yg M persists for many months. And, thus, a positive Yg M titer does not help to verify the age (freshness) of infection.
False negative test results are rare. Early antibody tests are usually negative because the immune response to B. burgdorferi
develops gradually. Experimentally infected dogs test positive in Elisa 4-6 weeks after infection. Titers remained at a high level for 3 months after infection. Increased titers almost always precede clinical lameness and fever in experimentally infected dogs, so a negative titer in animals with clinical signs is highly likely to rule out Lyme disease.
Since routine antibody tests (Elisa and ELISA), paired sample testing, Yg M and Yg G differentiation are not useful, other reliable tests such as Lesterne blot (immunoblotting is a method for detecting certain proteins) should be used. Or the new C6 Elisa. These tests have higher specificity and sensitivity than whole cell Elisa or ELISA and can be used to detect antibodies that specifically indicate Borrelia Burgdorferi infection. And to exclude vaccination or contact with other bacteria, Western blotting determines the spectrum of antibodies, and some antibodies have reactivity after natural infection that differs from that after vaccination. Following natural infection with B. burgdorferi, antibodies develop to several proteins, including outer surface proteins. One of these proteins, Osp C, appears when Borrelia in the host is exposed to warm temperatures, but is lost when the tick is exposed to low temperatures or during in vitro culture. Thus, Osp C is the major protein that responds to infection, and high amounts of antibodies to Osp C are produced after natural infection. In contrast, Osp A protein is produced by Borrelia while in arthropods (or during in vitro cultivation); thus, reactivity to Osp A, but not Osp C, occurs in vaccinated dogs (because vaccine strains are cultured in vitro) but is absent in naturally infected dogs. This effect is even more significant if a recombinant vaccine containing Osp A is used rather than whole Borrelia. Thus, Western blotting reflects the difference between vaccinated and naturally infected dogs.
The newest Elisa tests, which include Osp C, also help differentiate between natural and vaccine infections. Recently, the antigenic features of a 26-amino acid invariant region located within the central region of the molecule, Vis E, of the variable surface of Borrelia burgdorferi were studied. This region, called IR 6 (invariant region), has been identified as an antigenic constant between strains of the B. burgdorferi complex sensu lato and is immunodominant in both humans and dogs. A new, in-house produced Elisa was developed using a peptide sequence (C 6 ) biotinylated at the N-terminus in IR 6 and has recently become used in the USA (SNAP 3Dx, IDEXX, Portland, Maine). The antibody response to C 6 is highly specific for B. burgdorferi and is more sensitive than whole cell tests for early infection, detecting antibodies 3 weeks after infection. It is also more sensitive for detecting Borrelia infection than other commercial Elisa kits. Dogs with leptospirosis, spotted mountain fever, babesiosis, ehrlichiosis, and heartworm do not have antibodies to C6. Moreover, C6 is not affected by current Lyme disease vaccines. The level of C6 antibodies also correlates with the abundance of Borrelia, a rapid decline after antibiotic therapy, while the results of the commonly used Elisa remain at an average level for a long time, even after successful treatment. Although C 6 Elisa is already used in veterinary practice in endemic areas, it has not been consistent with control studies. It must be taken into account that both Western blotting and C 6 Elisa, although more specific, only indicate natural infection with B. burgdorferi and do not predict clinical disease.
Human CSF antibody titer to Lyme disease
compared with serum antibodies in making the diagnosis of neuroborreliosis. Intrathecal (interthecal, in the area of the spinal canal) production of specific antibodies to B.burgdorferi can be demonstrated if the ratio of CSF to serum antibodies is higher than the concentration of albumin, total Yg G against other infectious agents.
Increased intrathecal antibody concentrations have been observed in dogs with neurological impairment. The results of such reports are difficult to evaluate because the dogs were from endemic areas and there is no histopathological or cultural confirmation.
Cultivation of spirochetes from a sample of an ill patient is the definitive diagnosis, but most cases are difficult due to the low number of organisms present and insensitivity to isolation methods. Special media (modified Barbour-Stenner-Kelly medium) are required, but even then culture is insensitive. Skin manifestations are the most suitable tissue for intravital and postmortem culture when samples are taken at or near the site of tick attachment. Xenodiagnosis, in which uninfected ticks become infected after feeding on a suitable infected animal host, is reliable evidence when tested in laboratory tests, but is too time-intensive and is not used for routine diagnosis.
PCR is highly specific. The best material for diagnosis is skin samples, which should be taken as close as possible to the site of the tick bite. If the location of the bite is unknown, a sample should be taken near the joint where lameness or swelling was detected first on examination. If arthritis is not present, a sample should be taken from the site where ticks most commonly attach (usually the front of the dog. Urine can also be used for PCR, but is less sensitive. Blood is not used because Borrelia rarely spreads hematogenously. Joint fluid samples are synovia , or CSF is an excellent material for PCR if clinical signs are present. PCR does not distinguish between living and dead organisms; studies indicate that small fragments of Borrelia DNA may persist in synovial fluid after treatment and these fragments may give positive PCR results. However, experimentally injected Borrelia DNA (without m/o replication) was destroyed and undetectable after 3 weeks. The sensitivity of PCR is high, but samples must contain Borrelia DNA. A negative PCR result, therefore, never excludes the presence of Borrelia elsewhere in the body.
TREATMENT OF BORRELIOSIS IN ANIMALS
Because of the difficulty found in making an accurate diagnosis, antibiotics are often used empirically as a trial of therapy. There are many reports of successful recovery after a course of antimicrobial therapy in dogs with “diagnosed” Lyme arthritis. However, clinical improvements following any therapeutic intervention must be considered with caution because acute claudication and joint dysfunction are intermittent and often resolve within days to weeks regardless of antibiotics. Doxycycline is the drug of choice for tick-borne borelliosis, but it itself has a chondroprotective effect in non-infectious arthritis in dogs and thus leads to improvement in arthritis not associated with Lyme disease.
Antibiotics most effective for treating borreliosis
- these are tetracyclines, ampicillin or amoxicillin, intravenous cephalosporins of the 3rd generation and erythromycin and its derivatives. Doxycycline (given 10 mg/kg orally twice daily for 30 days) is the first choice because it is a fat-soluble tetracycline of relatively low cost. Other medications are usually reserved for persistent or chronic infections. Improvements often occur 24-48 hours after initiation of antimicrobial therapy. Significant success is achieved when used in the initial stage of a clinical disease. Research suggests that m/o is difficult to eliminate from animals with proven infection and that relapses occur despite the apparent adequacy of treatment. Most often, the treatment period is set at 30 days. However, based on scientific research, the purity of the body after 30 days of treatment is in question. Relapse may occur and PCR may become positive even after the end of antimicrobial therapy. Also, inflammatory changes that occur in various tissues, such as joints, can become persistent.
Neuroborreliosis: when the nervous system is affected
Due to the disease, at a certain stage, a sharp deterioration in the general condition of the animal is possible. The dog’s appetite disappears, and spontaneous development of complete or partial paralysis of the limbs is possible, with the hind legs most often affected. This condition can last quite a long time, and its symptoms may completely disappear.
If the nerve centers of the spinal cord or brain are severely damaged, disturbances in the motor activity of the animal’s body may be irreversible. This manifests itself as permanent paresis, paralysis or dysfunction of internal organs. The dog is subjected to severe suffering and often dies.
PROTECTION AGAINST TICK BORELLIOSIS
In the United States, vaccines based on whole cell killed bacteria and recombinant Osp A protein are commercially available. Whole-cell killed vaccines should be avoided because it is unsatisfactory that multiple vaccine components are not involved in protection against infection and that could potentially induce a delayed or perverse response. Hamsters immunized with the killed bacterium and exposed to infected mites develop arthritis over a period of weeks to months. This important circumstance hinders the development of the production of human vaccines based on whole bacteria. All human vaccines commercially available in the United States were based on recombinant DNA or derivatives of Osp, but even these have been removed from the market in Europe and other geographic areas; however, only whole cell killed vaccines are suitable for dogs (and there are no vaccines suitable for humans) because the multiplicity of infectious strains makes the development of recombinant product protection against different Borrelia species difficult. The multiplicity of infectious strains also makes the protection induced by the whole cell vaccine (currently on the market in Europe and containing B. burgdorferi sensu stricto strains) questionable, since cross-protection against B. burgdorferi species was not observed.
The advantage of tick-borne borelliosis vaccines based on the recombinant Osp A protein is that they induce antibodies only to Osp A, a protein that is produced in ticks and not in dogs. Vertebrate hosts infected with Borrelia through a tick bite rarely produce antibodies to Osp A. Osp A is produced by Borrelia in unfed ticks and changes to Osp C during vector transmission due to warm conditions while feeding on a vertebrate host. During feeding, antibodies to Osp A, which are present in the blood of the vertebrate due to vaccination, penetrate the tick and neutralize Borrelia before they enter the host. Antibodies to Osp A from a warm-blooded host cause growth arrest and invasion into the salivary glands of the tick. The spirochetes are either killed immediately by antibody-induced complement by lysis, or their motility is reduced so that they cannot continue their migration into the salivary glands of the tick. Vaccinated hosts have antibodies to Osp A and cannot bind Borrelia that produce Osp C. Thus, the risk of developing immune complex disease is reduced compared to whole cell vaccines.
A disadvantage of vaccination in general is that induced antibodies take several months to years to test positive in routine antibody tests. Western blotting or C6 ELISA should be used to distinguish vaccine antibodies from natural challenge. Additionally, vaccine deficiency is a possible hypersensitivity reaction that can develop if a dog is vaccinated that already contains the organism. Vaccinating an already infected dog does not clear the infection from the body or prevent clinical signs from appearing. Local and systemic allergic reactions have been reported with whole cell vaccines. The vaccine may also cause a breakdown in protection in the future, since Borrelia are known to change their genotype and phenotype and therefore survive despite m/o-specific antibodies. Vector control uses residual insecticides or growth regulators as a supportive measure, which can help reduce the incidence of infection in humans and domestic animals. Collars impregnated with amitraz help reduce the transmission of spirochetes.
What are the conclusions?
Now you know the answers to questions about whether dogs get borreliosis and what you should do about it. Remember: infection is possible only after a tick bite. Your pet cannot get infected from another animal. Likewise, borreliosis is not transmitted from a dog to a person, except in cases where the blood of a sick animal can get into an open wound.
Symptoms Revisited: Watch for signs of intermittent claudication accompanied by fever, lack of appetite, and general depression. Pay attention to signs of partial or permanent paralysis.
https://www.youtube.com/watch?v=QoWf2Gpu0ak
If the disease is not advanced, then provided treatment is started on time, the prognosis can be considered favorable. Diagnosing yourself and trying to help your pet on your own is an unproductive activity. If you suspect a disease, the only way out is to go to the clinic and see a veterinarian as soon as possible.
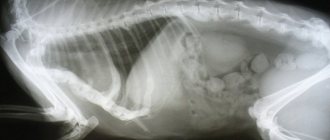
BORRELIOSIS IN CATS
Antibodies positive for borreliosis were found in cats
and experimental infection has been reproduced, but naturally occurring disease has not been documented. Approximately 13% of cats tested in the US had antibodies, but there was no difference in positivity between cats with and without lameness. In the UK, 4% of cats had antibodies. Cats may be more resistant than dogs to developing clinical signs. However, when cats were experimentally inoculated with microorganisms directly from arthropods, they exhibited multiple lameness and, at necropsy, had inflammation in the joints, lungs, lymph nodes and central nervous system. Arthritis and meningitis dominated.
Bartonellosis: what is it and where is it common?
“Cat scratch disease” is closely related to climatic conditions. It has been established that where fleas feel free, the incidence of bartonellosis is higher. This is natural, the greater the percentage of sick cats, the theoretically they can be carriers of a disease dangerous to humans.
In cold countries, bacteremia is at the level of 0-3%, in St. Petersburg – 4%. In warm climates, bartonellosis infection reaches 68% (Philippines), 93% (nursery in the USA). 6 species of Bartonella are considered the most dangerous, although there are at least 24 species and 14 of them are zoonotic.
PUBLIC HEALTH IN LYME DISEASE
It is not clear that domestic dogs and cats infected with tick-borne borelliosis pose a direct risk to humans, excluding the introduction of unfed ticks into the house. Ticks do not survive in the house for a long time, and if the tick is engorged, it will not transfer to another host without molting. However, a partially fed tick may feed again and thus carry a risk of infection since it requires a short period of attachment. Direct horizontal transmission from dogs and cats to humans is not possible. It is believed that urine from an infected dog can be a source of infection for humans. Borrelia, however, is rapidly destroyed in urine, and there is no definitive evidence that human infections have occurred after contact with infected dogs. Additionally, recent studies in the Netherlands found no positive correlation between the presence of antibodies in hunters compared to their dogs. Although Lyme disease is classified as a zoonosis, dogs, cats and humans are incidental hosts for the wild cycle that exists in nature. Lyme borreliosis in humans
usually associated with external activity that increases the likelihood of tick exposure.
^Top
If your kidneys are damaged
Disturbances in the functions of urination and urination caused by borreliosis in dogs manifest themselves in the form of frequent urination in small portions, accompanied by pain and traces of blood in the urine. Without treatment of this important organ, glomerulonephritis may occur with subsequent development of renal failure.
Symptoms in this case are reduced to diarrhea and vomiting, a sharp decrease (up to complete absence) of appetite, weight loss, thirst and increased urination. You can also often observe the development of subcutaneous edema in the abdominal cavity and on the surface of the thighs on the inside.
We emphasize once again that this disease is characterized by different symptoms in each individual case. Thoroughbred animals are much more likely to suffer from nervous system disorders and joint problems. Outbred dogs are more susceptible to kidney disease. In addition, any of the above complexes may be present either by itself or together with others.
Good to know
- Trichopolum instructions for veterinary medicine
- Instructions for the use of the antibiotic Baytril in animals
- Instructions for use of metronide
- Instructions for ceftriaxone preparations for animals
- Use of Metrogyl in veterinary medicine
- Instructions for doxycycline in animals
- Metronidazole (Metronidazole) for animals (instructions for use in veterinary medicine, doses, indications and contraindications)
- Atovaquone (ATOVAQUONE)
- Azithromycin, instructions for animal therapy